Amgen's GLP1: MariTide (formerly AMG-133)
Amgen is working on a new GLP1, entering the race -- AM-133. We dig into the trials and research and see if it's got any promise.

Just about every pharmaceutical company big enough to do so is attempting to get in on the GLP1 Receptor Agonist race – companies are producing new methods, compounds and ways of using GLP1 Receptor agonists.
Check out our quick explainer
What is Amgen?
Amgen (which used to be short for Applied Molecular Genetics) is an American pharmaceutical company started in 1980:

Similar to some of it's competitors in the GLP1 space (Novo Nordisk, Eli Lilly), it started with a focus on insulin.
While Amgen is much younger than those other competitors, it's certainly made great discoveries, including Neupogen which preevents infections in patients with under-active immune systems due to chemotherapy.
This is to say that Amgen clearly has the drug discovery and testing ability to produce new GLP1s, and are worth looking at as a serious competitor in the space.
You can read more about Amgen at their website:

What is MariTide (AMG-133)?
Despite the somewhat non-organic name, AM-133 is a new GLP1 receptor agonist – a peptide-based molecule delivered by subcutaenous injection.
In addition to being a GLP1 Receptor Agonist, AMG-133 is also a GIP inhibitor – this is different from how Tirzepatide (Zepbound, Mounjaro) works – they agonize GIP along with GLP1.
Wondering what the difference between GLP1 and GIP is? We've put together an explainer:

In fact, there are also triple agonists like the new Retatrutide which also agonizes the GCGR receptor):

It's interesting that AMG-133 now
What's also interesting about AMG-133 is that it's a once monthly dose – dramatically easy to take and manage than the current suite of GLP1s.
Amgen looks to have found a novel way to the success that is offered by drugs like Retratrutide, and has what seems to be a solid entry into the GLP1 RA market.
We do also have a nice visualization of the drug:

What does the research say?
Information on Amgen is relatively hard to find – Amgen described some of the initial results of trials as early as 2022, back when AMG-133 was in Phase 1:

You can find the details of the trial here:
The results were published and are available on PubMed:

The trial is very small – only 110 participants were present, but we'll go through the results regardless, and unfortunately the results weren't properly published, so we can only go on what Amgen has published, and the details published at the link above.
- Participants had a BMI of ≥30.0 kg/m² and ≤40.0 kg/m².
- Average age of participants that received AMG-133 were 47 years old
- Majority of negative side effects were gastrointestinal (similar to other GLP1 RAs)
- Weight loss was maintained up to 150 days after the last dose
The research contained various great diagrams comparing outcomes for placebo to various dosages of AMG-133:
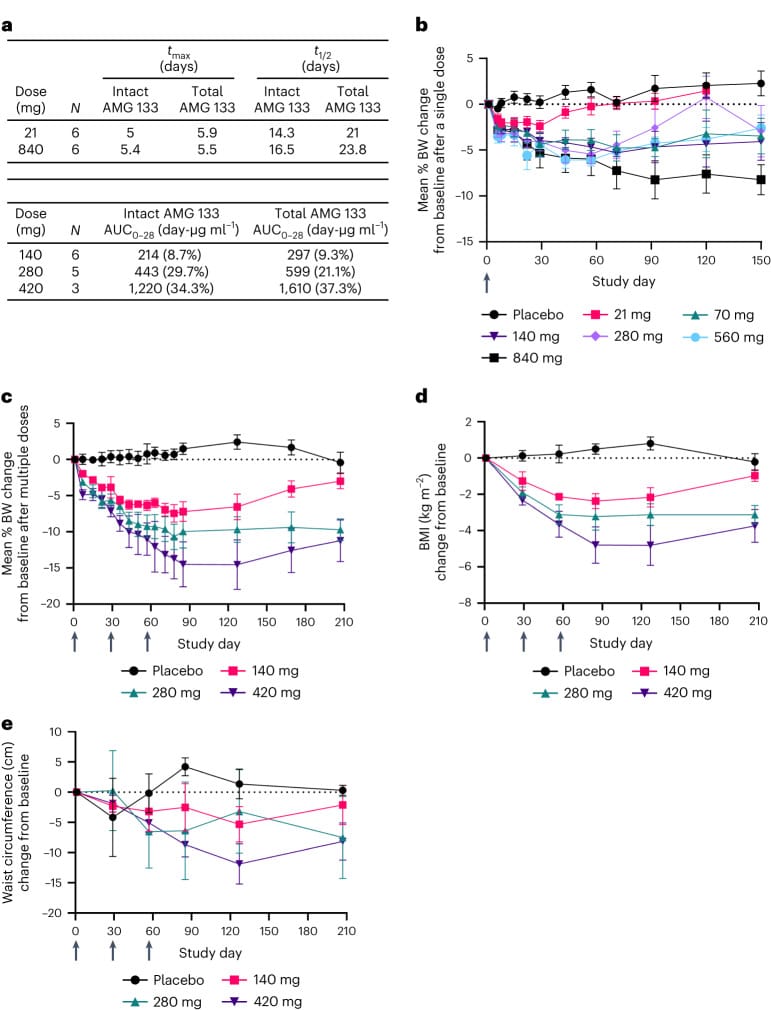
One interesting insight from the study was the occurrence of negative side effects – they decreased dramatically after the first dose:
Of the participants who received at least two doses, 68% of them had GI-related TEAEs after the first dose and only 9% of participants had GI-related TEAEs after subsequent doses.
As we've noticed here, the researchers were also careful to note the size of the study being a major factor:
The main limitation of this FIH study was the small sample size. Cautious interpretation of PD effects, including metabolic parameters is thus required. Furthermore, the participants had neither diabetes nor high triglyceride and cholesterol levels, which further limits the generalizability of the metabolic parameter findings.
The primary endpoints of this study were more about safety than weight loss, so the study spends much tie talking about the development of the molecule and related effects, and the safety of AMG-133 versus other alternatives (GLP1s).
How are AMG-133 Phase 2 trials progressing?
It looks like Amgen is going "all-in" on AMG-133 (now called Martide) – the company has removed another obesity drug that was under development to focus fully on AMG-133.
That said, AMG-133 is still in Phase 2 trials – and only company insiders have been able to get access to results:
Members of Amgen leadership have viewed an interim analysis of the phase 2 study.
“Following the interim analysis, I would say we're confident in MariTide’s differentiated profile and believe that it will address important unmet medical needs,” Amgen CEO Bob Bradway said on the call.
We can only expect/wait on results from the Phase 2 trials being shared later in 2024:
Amgen expects to share top-line 52-week data from the 11-arm phase 2 study in late 2024, according to Bradner.
For now, all we can do is wait and watch with excitement for when the next set of results will be published with regards to MariTide. The development of MariTide is likely to increase availability of GLP1s and accessibility for those with type 2 diabetes and weight loss management meeds.









